#1 What Is Metal Corrosion
Metal Corrosion refers to the destruction of metals under the chemical action of environmental media and the joint action of physical, mechanical or biological factors, that is, the damage of metals under the action of its environment.
This issue mainly introduces the corrosion of metals in different environments, including the corrosion in the atmosphere, sea water, soil, industrial environment and human environment.
#2 The Corrosion Of Metals In The Atmosphere
1.The Overview Of Atmospheric Corrosion
The damage of metal materials or structures caused by chemical or electrochemical reactions under atmospheric conditions is called atmospheric corrosion. Atmospheric corrosion is a common corrosion phenomenon. The amount of steel used in the atmosphere worldwide typically exceeds 60% of its production. For example, steel beams, rails, various mechanical equipment, vehicles, etc. are used in the atmospheric environment. The metal loss due to atmospheric corrosion accounts for more than 50% of the total corrosion. Therefore, it is very necessary to understand and study the mechanism, influencing factors and prevention methods of atmospheric corrosion.
2.The Atmospheric Corrosion Classification
The main components of the atmosphere are almost constant on a global scale, and the moisture content in it will vary with conditions such as regions, seasons, and time. Participating in the atmospheric corrosion process is oxygen and water vapor, carbon dioxide. Atmospheric corrosion is divided into three categories according to the degree of moisture on the metal surface:
- Dry atmospheric corrosion. Dry atmospheric corrosion is corrosion in the absence of a liquid film layer on the metal surface. It is characterized by the formation of an invisible protective oxide film (1~10nm) and some metal tarnish on the metal surface. Such as copper, silver, etc., a layer of film formed in the air polluted by sulfide.
- Tidal atmosphere corrodes. Moisture atmospheric corrosion refers to the corrosion of metals in an atmosphere with a relative humidity of less than 100%, where there is a thin liquid film layer (10nm~1μm) invisible to the naked eye on the surface. If iron is not exposed to rain, it will rust.
- Humid atmosphere corrosion. Wet atmospheric corrosion refers to the corrosion of metals with a relative humidity greater than 100%, such as water splashed directly on the metal surface in the form of rain, fog, water, etc., and there is a water film (1μm ~ 1mm) visible to the naked eye on the surface.
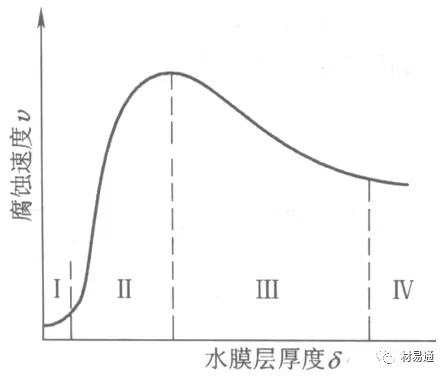
According to the above figure, explain the law of corrosion rate and water film thickness:
- In area I, the metal surface has only a few water molecules thick (1~10nm) water film, and no continuous electrolyte solution has been formed, which is equivalent to dry atmospheric corrosion. Corrosion rate is small.
- When the thickness of the water film on the metal surface in area II is about 1 μm, due to the formation of a continuous electrolyte layer, the corrosion rate increases rapidly, and atmospheric corrosion of moisture occurs.
- Area III When the thickness of the water film increases to 1 mm, wet atmospheric corrosion occurs, and it is significantly difficult for oxygen to diffuse to the metal surface through the film, so the corrosion rate decreases significantly.
- The thickness of the water film on the metal surface in area IV is greater than 1mm, which is equivalent to the corrosion of fully immersed in the electrolyte, and the corrosion rate is basically unchanged.
Commonly referred to as atmospheric corrosion refers to corrosion in humid air at room temperature.
3.Atmospheric Corrosion Mechanism
Atmospheric corrosion is characterized by the corrosion process of the metal surface under a thin layer of electrolyte, and the corrosion law conforms to the electrochemical corrosion law.
The cathodic process of atmospheric corrosion is dominated by oxygen depolarization when a continuous thin layer of electrolyte is formed on the metal surface.
Metals such as iron and zinc are fully immersed in reducing acid solution. The cathodic process is mainly hydrogen depolarization. Under the acidic water film formed by the urban polluted atmosphere, the corrosion of these metals is mainly oxygen depolarization corrosion.
Under the thin liquid film, the anode process is greatly hindered, and oxygen is more likely to reach the metal surface, forming an oxide film or an oxygen adsorption film, making the anode in a passive state. Anode passivation and metal ionization process difficulties lead to anode polarization.
When the liquid film thickens, which is equivalent to wet atmospheric corrosion, there is a diffusion process for oxygen to reach the metal surface, and the corrosion process is controlled by the oxygen diffusion process.
The damp atmospheric corrosion is mainly controlled by the anode process; the wet atmospheric corrosion is mainly controlled by the cathodic process.
Atmospheric corrosion conditions are different, and the composition and structure of the rust layer are very complex. Evans believes that the rust layer of atmospheric corrosion is under humid conditions, the rust layer acts as a strong oxidant, and the anodic reaction occurs at the metal/Fe3O4 interface in the rust layer:
- The reduction reaction of Fe3+→Fe2+ occurs in the rust layer, and the rust layer participates in the cathodic process.
- When the rust layer is dry and the relative humidity of the external air drops, the rust layer and the base steel at the bottom are re-oxidized to Fe3+ oxides under the action of oxygen in the atmosphere, and the rust layer accelerates steel corrosion under the condition of alternating dry and wet conditions process. • Carbon steel rust layer structure is generally divided into inner and outer layers:
- The inner layer is close to the interface of steel and rust, with good adhesion and dense structure, mainly composed of dense grains with a little Fe3O4 and amorphous FeOOH;
- The outer layer is composed of loose crystalline α-FeOOH and γ-FeOOH.
4.Metal Corrosion Characteristics In Industrial Atmosphere
SO2, NO2, H2S, NH3 in the industrial atmosphere all increase the corrosion of the atmosphere and accelerate the corrosion rate of metals.
Petroleum, coal and other combustion waste gases contain the most SO2, and the content of SO2 in cities and industrial areas can reach 0.1~100mg/m3.
The average corrosion rate of commonly used metals in different atmospheric environments.
- 1) When the air is very pure, the corrosion rate is very small, and it only increases slightly with the increase of humidity.
- 2) In polluted air, when the relative humidity of the air is lower than 70%, even if it is exposed for a long time, the corrosion rate is very slow. However, in the presence of SO2, when the relative humidity is slightly higher than 70%, the corrosion rate increases sharply.
- 3) Air polluted by ammonium sulfate and soot particles accelerates metal corrosion.
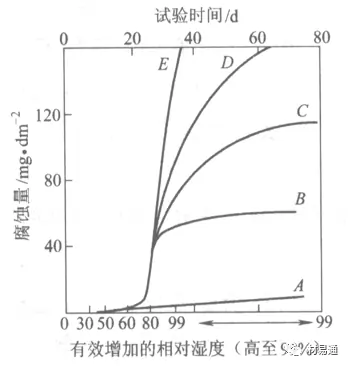
In the figure, A-pure air, B-with (NH4)2SO4, C-0.01%SO2, D-(NH4)2SO4+0.01%SO2, E-smoke particles+0.01%SO2.
- Polluted Air, lower than the critical humidity, no water film on the metal surface, the metal is corroded by chemical action, and the corrosion rate is very small. Above the critical humidity, the formation of water film, electrochemical corrosion, and a sharp increase in corrosion rate.
- The effect of SO2 in the atmosphere on metals that are not resistant to H2SO4 corrosion, such as Fe, Zn, Cd, Ni is very obvious. rising in a straight line.
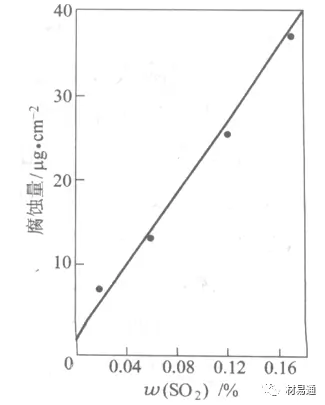
4.The Mechanism Of Metals SO2 Promoting Atmospheric Corrosion
- The corrosion mechanism of SO2 is an autocatalytic process of sulfate caverns.
- The mechanism of SO2 promoting atmospheric corrosion of metals, there are two main ways:
- One is that part of SO2 can be directly oxidized into SO3 in the air, and SO3 dissolves in water to form H2SO4;
- Second, a part of SO2 is adsorbed on the metal surface, reacts with Fe to form soluble ferrous sulfate, FeSO4 is further oxidized and generates H2SO4 due to strong hydrolysis, and H2SO4 reacts with Fe to accelerate corrosion in this cycle.
- The whole process has an autocatalytic effect, that is, the effect of the so-called sulfate cavern in the rust layer.
The protective ability of a rust layer is influenced by the conditions prevailing at the time of its formation. If the generated rust layer is attacked by sulfate, the rust layer has almost no protective ability. On the contrary, if the initial rust layer is rarely polluted by sulfate, its protection is better.
5.Factors Affecting Atmospheric Corrosion and Corrosion Prevention Methods
• Humidity
Humidity is an important factor in determining the type and rate of atmospheric corrosion. The atmospheric relative humidity value at which the atmospheric corrosion rate begins to increase sharply is called the critical humidity. For iron, steel, copper, and zinc, the critical humidity is between 70% and 80%. It can be seen from Figure 7 that the humidity is less than the critical humidity, the corrosion rate is very slow, and there is almost no corrosion. If the humidity is reduced below the critical humidity, atmospheric corrosion of metals can be prevented.
• Atmospheric Composition
The geographical environment is different, and there are impurities such as SO2, H2S, NaCl and dust. They accelerate corrosion to varying degrees. Especially SO2. Exhaust gas from coal and oil combustion contains a large amount of SO2. In winter, fuel consumption is high, SO2 pollution is more serious, and the impact on corrosion is even greater.
Iron, zinc and other metals form easily soluble sulfate compounds in the SO2 atmosphere, and their corrosion rate increases in a linear relationship with the SO2 content in the atmosphere.
There are many tiny NaCl particles in the ocean atmosphere, they fall on the surface of the metal, have a hygroscopic effect, and increase the conductivity of the surface liquid film layer, and the chloride ion itself has a strong turbidity, thus making corrosion become more serious.
Solid particles in the atmosphere are called dust. Its composition is complex. In addition to sea salt particles, there are solid particles such as carbon and carbides, silicates, nitrides, and ammonium salts. The dust content in the urban atmosphere is about 2mg/m3, and the dust in the industrial atmosphere can even reach more than 1000mg/m3.
Dust has the following effects on the atmosphere:
- 1) Dust itself is corrosive, such as ammonium salt particles can melt into the water film on the metal surface, increasing the conductivity or acidity to promote corrosion.
- 2) Dust itself has no corrosive effect, but it can absorb corrosive substances, such as carbon particles can absorb SO2 and water vapor to form a corrosive acidic solution.
- 3) Dust deposits on the metal surface to form gaps and condense water, forming oxygen concentration differences to cause crevice corrosion.
Metal components and instruments and equipment exposed to the atmosphere should be dust-proof.
6.The Methods To Prevent Atmospheric Corrosion:
- Improve the corrosion resistance of metal materials Adding Cu, P, Cr, Ni and rare earth elements to carbon steel can improve its atmospheric corrosion resistance. For example, the Cor-Ten steel (Cu-P-Cr-Ni low-alloy steel) in the United States has 4-8 times the atmospheric corrosion resistance of carbon steel.
- Organic and inorganic coatings and metal coatings are used.
- Use gas phase corrosion inhibitor.
- Reduce atmospheric humidity, mainly used for the protection of storage metal products.
Rational design components. Preventing water in the gaps, removing dust on metal surfaces, etc. are beneficial to anti-corrosion, environmental protection, and reducing air pollution. Not only is it beneficial to people’s health, but it is also very important to prolong the service life of metal materials in the atmosphere.
#3 The Corrosion Of Metals In Seawater
1.The Overview of Seawater Corrosion
The ocean covers 70% of the earth’s surface, and seawater is the largest and corrosive natural electrolyte in nature. my country’s coastline is as long as 18,000km, and the sea area is vast. Factories in coastal areas often use seawater as the cooling medium. Under the action of seawater, the cast iron pipes of the cooler can only be used for 3 to 4 years; the cast iron impeller of the seawater pump can only be used for about 3 months; the corrosion rate of the inner wall of the carbon steel cooling box Up to 1mm/a or more. In recent years, marine development has been paid attention to, and metal components such as marine transportation tools, offshore oil production platforms, mining and underwater transportation and storage equipment are increasingly threatened by seawater and marine atmospheric corrosion; research on metal corrosion and its protection in marine environments is of great importance. Significance.
2.The Characteristics Of Seawater Corrosion
• Salt and conductivity
Seawater is a corrosive medium, and it is characterized by containing a variety of salts. The salt content is mainly NaCl. Seawater is often regarded as a NaCl solution with a mass fraction of 3% or 3.5%.
Salinity refers to the total grams of dissolved solid salts in 1000g of seawater. Generally, the salinity of seawater is between 3.2% and 3.75%, and 3.5% is usually taken as the average salinity of seawater. The content of chloride ions in seawater is very high, accounting for 58.04% of the total salt content, making seawater highly corrosive.
The average conductivity of seawater is 4×10-2S/cm, far exceeding that of river water and rainwater.
.Dissolved oxygen
Dissolved oxygen in seawater is an important factor for seawater corrosion. Under normal circumstances, the surface layer of seawater is saturated with air, and the concentration of oxygen varies with water temperature in the range of (5~10)×10-6cm3/L. It can be seen from the table that the higher the concentration of salt and the higher the temperature, the smaller the solubility of oxygen.
.Electrochemical properties of seawater
- For most metals, except the particularly active metal magnesium and its alloys, the corrosion process in seawater is an oxygen depolarization process, and the corrosion rate is controlled by the oxygen diffusion process.
- When most metals (iron, steel, zinc, etc.) are corroded in seawater, the blocking effect of the anode process is very small, and the concentration of Cl- ions in seawater is high. In seawater, the method of increasing anode blocking is used to reduce seawater corrosion It is unlikely that the addition of alloying element molybdenum can inhibit the damage of Cl- to the passivation film and improve the corrosion resistance of the material in seawater.
- The conductivity of seawater is very high, and the resistive block is very small. For seawater corrosion, the activity of the micro-battery is greater, and the activity of the macro-battery is also greater. In seawater, the galvanic corrosion caused by the contact of dissimilar metals has a considerable destructive effect. For example, the bronze propeller of a ship can cause corrosion of the steel ship hull at a distance of tens of meters.
- Metals in seawater are prone to localized corrosion damage. Such as pitting corrosion, crevice corrosion, turbulent corrosion and cavitation corrosion.
3.Factors Affecting Seawater Corrosion
Salts in seawater, dissolved oxygen, marine organisms and decaying organic matter, seawater humidity, flow rate and pH value all have a great influence on seawater corrosion.
1) Salt.
Mainly NaCl, the concentration of salt in seawater is similar to the salt concentration range where the corrosion rate of steel is the highest. When the concentration of dissolved salt exceeds a certain value, the corrosion rate of metal will decrease due to the decrease of oxygen solubility.
2) pH value.
The pH value of seawater is between 7.2 and 8.6. The pH value can change slightly due to photosynthesis; in the deep sea, the pH value decreases slightly, which is not conducive to the formation of a protective salt film on the metal surface.
3) Dissolved oxygen.
Dissolved oxygen in seawater is an important factor for seawater corrosion. Corrosion of most metals in seawater is controlled by oxygen depolarization.
- The dissolved oxygen content varies with the depth of seawater.
- The oxygen content of seawater surface in contact with the atmosphere is as high as 12×10-6.
- From sea level to -800m depth, the oxygen content gradually decreases and reaches the lowest value;
- Marine animals consume oxygen;-800m and then down to -1000m, the amount of dissolved oxygen rises again, close to the oxygen concentration of the seawater surface, because the temperature of deep seawater is lower and the pressure is higher.
4) Temperature.
For every 10°C increase in seawater temperature, the chemical reaction rate increases by about 10%, and the corrosion rate of metals in seawater will increase accordingly. However, as the temperature rises, the solubility of oxygen in seawater decreases. For every 10°C increase, the solubility of oxygen decreases by about 20%, which slightly reduces the corrosion rate of metals.Temperature changes are associated with marine life. The relationship between seawater temperature and metal corrosion rate is quite complex.
5) Flow rate.
Corrosion of many metals has a great relationship with seawater velocity. Especially for commonly used metals such as iron and copper, there is a critical flow rate. When the flow rate exceeds this rate, metal corrosion will be significantly accelerated. However, stainless steel containing titanium and molybdenum has better corrosion resistance in high-velocity seawater
6) Marine life
Marine organisms attach to the surface of ships or offshore structures to form crevices, which can easily induce crevice corrosion.
• The physiological action of microorganisms will produce corrosive substances such as ammonia, CO2 and H2S. For example, the action of sulfate-reducing bacteria will produce S2-, which will accelerate metal corrosion.
#4 The Corrosion Resistance Of Common Metal Materials In Seawater
The corrosion resistance of metal materials in seawater varies greatly. The best corrosion resistance is titanium alloy and Ni-Cr alloy, while the corrosion resistance of cast iron and carbon steel is poor. Although the uniform corrosion rate of stainless steel is very small, it is prone to pitting corrosion in seawater.
Measures to prevent seawater corrosion
- Development and application: seawater corrosion-resistant materials; such as titanium, nickel, copper and their alloys, seawater-resistant steel (Mariner).
- Cathodic protection: It is more reasonable to use protective screen protection in the most severely corroded parts, and the simple and feasible sacrificial anode method can also be used.
- Coating: In addition to the application of anti-rust paint, double anti-fouling paint can also be used to prevent biofouling. Some fixed steel structures in tidal and splash areas can be covered with Monel alloy.
#5 The Corrosion Of Metals In Soil
1.Overview Of Soil Corrosion
Soil is an extremely complex heterogeneous multiphase system composed of various components such as soil particles, aqueous solution, gas, organic matter, charged colloidal particles, and mucus colloids. Due to the uneven composition and performance of the soil, it is very easy to form an oxygen concentration cell corrosion, which causes severe local corrosion of underground metal facilities. Oil, gas, water pipelines and cables buried in the ground leak oil, gas or water due to perforation. or cause telecommunications equipment to malfunction. These are often difficult to overhaul, causing great loss and hazards.
Soil corrosion is a very important form of corrosion. In advanced countries, there are more than one million kilometers of underground oil and gas, and the cost of replacing various pipes due to corrosion damage is as much as hundreds of millions of dollars every year. With the development of the petroleum industry, it is of great practical significance to study the laws of soil corrosion and find effective anti-corrosion methods.
2.The Characteristics Of Soil Corrosion
soil properties
- Soil heterogeneity. Soil is a complex multiphase system composed of soil particles, water, air, organic matter and other components. The actual soil is generally a combination of these different components in a certain proportion.
- Soil conductivity. Since water in soil can exist in various forms, there is always a certain amount of water in the soil, so the soil is conductive. Soil is also an electrolyte. The porosity and water content of the soil affect the air permeability and electrical conductivity of the soil.
- Soil inhomogeneity. Oxygen in the soil is dissolved in water and exists in the gaps in the soil. The oxygen concentration in the soil is closely related to the moisture and structure of the soil. The oxygen content is the highest in the dry sandy soil, followed by the moist sandy soil, and the least in the moist and dense clay. This is what causes the corrosion of oxygen concentration cells.
- The acidity and alkalinity of the soil.
Most soils are neutral, with a pH between 6.0 and 7.5. • Some soils are alkaline, such as the saline-alkali soil in Northwest my country with a pH value of 7.5~9.0; • Some soils are acidic, such as humus soil and swamp soil with a pH value of 3~6. It is generally believed that the lower the pH value, the more corrosive the soil.The corrosion of most metals in soil belongs to oxygen depolarization corrosion. The corrosion of metals in soil is essentially the same as that in electrolytes.Anodic process:
- The anode reaction rate is mainly controlled by the difficulty of the metal ionization process.
- In soils with low pH, there is very little OH-. Due to the inability to generate Fe(OH)2, the concentration of Fe2+ ions increases in the anode region.
- Fe(OH)2 produced in neutral and alkaline soils has very little solubility, and precipitates on the steel surface, which has a certain retardation effect on anode dissolution.
- The soil contains carbonates, which may form insoluble deposits on the surface of the anode and act as a protective film.
- Chloride ions and sulfate ions in the soil can form soluble salts with Fe2+ ions to accelerate the dissolution of the anode.
Cathode process:
- In weakly acidic, neutral and alkaline soils, the cathodic reaction is mainly the depolarization of oxygen.
- The water dissolved oxygen in the soil is limited, and the main effect on soil corrosion is the oxygen in the crevice and capillary.
- The transfer process in soil is more complex and slower. In wet cohesive soils, oxygen transfer is rather difficult due to poor water seepage capacity and air permeability, hindering the cathodic process. When the pH value of soil moisture is greater than 5, corrosion products can form a protective layer.
3.The Forms Of Soil Erosion
Corrosion caused by uneven inflation
This kind of corrosion mainly refers to the macroscopic battery corrosion caused by the difference in oxygen concentration when underground pipelines pass through different geological structures and soil zones with different moisture levels.
Corrosion caused by stray current
Stray current is a leakage phenomenon. High-power electrical devices for direct current, electric railways, electrolysis and electroplating, electric welding machines and other devices.
If the insulation between the rail and the soil is poor, some of the current will leak from the rail to the soil. Metal pipes are buried near the rails, and the stray current enters the metal pipes through the soil, and then returns to the power supply through the soil and the rails. This is equivalent to two macroscopic battery functions: rail-anode, soil-electrolyte, pipe-cathode, pipe-anode, soil-electrolyte, rail (ground)-cathode.
Corrosion caused by microorganisms
- There are not many bacteria that have an effect on corrosion, the most important of which are sulfate bacillus and sulfate reducing bacteria (anaerobic bacteria).
- These two kinds of bacteria can reduce sulfate in the soil to produce S2-, a small part of which is consumed in the metabolism of the microorganism itself, and most of it can be used as a cathode depolarizer to promote corrosion reactions.
- When the pH value of the soil is 4.5~9.0, the growth of sulfate-reducing bacteria is most suitable;
- When 11 <pH value<3.5, the activity and growth of this kind of bacteria are very difficult.
The measures to prevent soil corrosion
- Use paint or cover glass cloth for waterproofing;
- Electrochemical protection is used, and the sacrificial anode method is mostly used, and the combined use of cathodic protection and coating is better;
- Using metal coating or cladding metal, galvanized layer, etc.
#6 The Corrosion Of Metals In Industrial Environments
01 Corrosion Of Metals In Acid Solutions
- Acid is a general term for a class of compounds that can ionize in aqueous solution to form H3O+ ions. H+ is generally used to represent H3O+.
- Oxidizing acids corrode metals quite differently from non-oxidizing acids.
- In the corrosion process, the characteristic of non-oxidizing acid is that the cathodic process of corrosion is basically a hydrogen depolarization process, and increasing the acidity of the solution will correspondingly increase the cathodic reaction and increase the metal corrosion rate.
- Oxidizing acids are characterized by the cathodic process being mainly the reduction of the oxidant causing metal corrosion (eg nitrate reduction to nitrite). However, when the concentration of oxidizing acid exceeds a certain critical value, the passivation metal enters a passive state and corrosion is inhibited. The corrosiveness of acid solution is related to the strength of the acid on the one hand, and also related to the oxidation ability of the anion of the acid.
- Industrial inorganic acids include sulfuric acid, nitric acid, and hydrochloric acid, which cause corrosion damage to equipment and cause serious economic losses.
Corrosion of metals in sulfuric acid:
- High concentration of H2SO4 is a strong oxidizing agent, which can make metals with passivation ability enter a passive state, while low concentration of H2SO4 has no oxidation ability and is very corrosive.
- The corrosivity of sulfuric acid mainly depends on the temperature and concentration. The oxidant and flow rate can also affect the corrosivity of sulfuric acid to various materials.
- Industrial materials resistant to sulfuric acid are cheap carbon steel, lead and lead alloys. Figure 11 and Figure 12 respectively show the relationship between the corrosion rate of iron and lead and the mass fraction of sulfuric acid.
The relationship between the corrosion rate of iron and the mass fraction of sulfuric acid
- When the mass fraction of sulfuric acid is lower than 50%, the corrosion rate of carbon steel increases sharply with the increase of concentration;
- When the mass fraction exceeds 50%, the corrosion decreases sharply as the concentration increases;
- When the mass fraction exceeds 70%, carbon steel hardly corrodes. A sulfate (FeSO4) protective film is formed on the steel surface. Therefore, steel materials can be used for storage tanks and transportation pipelines for the production of concentrated sulfuric acid with a mass fraction exceeding 70%.
- When the mass fraction exceeds 101% of medium-temperature fuming H2SO4, two problems should be paid attention to: ①Concentrated H2SO4 is a strong water-absorbing agent; ②The sulfate protective film (FeSO4) is easily damaged.
- When the mass fraction of sulfuric acid is lower than 70%, equipment made of lead should be used, and a protective film of PbSO4 can be formed on the lead surface. In sulfuric acid environments, steel and lead are complementary.
Corrosion of metals in hydrochloric acid
- • HCl strong acid, except for a few metals such as silver and titanium, most metals or alloys cannot form insoluble metal salt films in HCl.
- • Cl- in HCl is extremely strong, except for a few metals with excellent passivity such as titanium, the passivation film on the metal surface is pitted due to the damage of chloride ions in hydrochloric acid.
•a Relationship between hydrochloric acid concentration and corrosion rate
The corrosion rate of industrial pure iron and carbon steel increases exponentially with the concentration of hydrochloric acid, and steel cannot be used in hydrochloric acid medium.
Influence of Dissolved Oxygen or Oxidant in Hydrochloric Acid on Corrosion
• When oxidizing agent exists in HCl, the corrosion rate of copper, molybdenum and nickel-based alloys increases significantly. When the concentration of HCl is high, the cathode is hydrogen depolarized, but when the concentration of hydrochloric acid is low, oxygen depolarization dominates and the corrosion rate increases.
Corrosion of Passive Metals in Hydrochloric Acid
• For metal materials that can be passivated by electrochemical or chemical methods, their passivation zone is very narrow or does not exist in HCl. Therefore, metal materials resistant to HCl corrosion are limited to special metals and alloys with strong passivation properties, such as Ta, Zr and Ti-Mo alloys.
#7 Materials Resistant To Hydrochloric Acid Corrosion
1.Ti-Mo alloy
• The development of corrosion-resistant Ti alloy is to improve the corrosion resistance of pure Ti in strong reducing medium. W. L. Finlay found that Ti-Mo alloy has excellent corrosion resistance to strong reducing sulfuric acid and hydrochloric acid. •The corrosion rate of Ti-30~40Mo alloy in boiling HCl with a mass fraction of 20% is 10mm/a; •Industrial pure titanium can only be used in room temperature HCl with a mass fraction of 3%~10% Currently Ti- 30Mo and Ti-32Mo are the most corrosion-resistant Ti alloys in reducing acids, and do not contain rare and precious metals, so they are widely valued.
2.Corrosion Behavior of Different Steels in Nitric Acid
1) Carbon steel
- • When the mass fraction of nitric acid is lower than 30%, the corrosion rate of carbon steel increases with the increase of acid concentration, and the corrosion rate reaches the maximum value when the mass fraction is around 30%.
- • When the mass fraction exceeds 30%, the corrosion rate drops rapidly;
- • When the mass fraction reaches 50%, the corrosion rate is the smallest, indicating that the steel is passivated. • When the mass fraction exceeds 80%, the corrosion rate of carbon steel increases sharply again, and the steel appears overpassivated and dissolved.
- • Iron or steel is suitable for HNO3 concentration in the range of 30%~80% (mass fraction).
2) Stainless steel.
It has good corrosion resistance to nitric acid. Figure 4-17 is a corrosion diagram of 18-18 stainless steel in nitric acid. Stainless steel is a corrosion-resistant material widely used in nitric acid systems, such as ammonium nitrate and most equipment in the production of nitric acid are made of stainless steel. Overpassivation corrosion is prone to occur in hot nitric acid with a concentration exceeding 70%.
3) Al and its alloys.
It has good corrosion resistance to fuming HNO3 at medium temperature, and when [HNO3]<85%, the corrosion resistance of Al is not good at room temperature.
4) High silicon cast iron.
Si mass fraction > 13% alloy cast iron is called high silicon acid-resistant cast iron. It has good corrosion resistance to various inorganic acids including hydrochloric acid.
•The mass fraction of Si in high-silicon cast iron generally does not exceed 15%, otherwise a metastable brittle η phase (Fe5Si2) will be generated.
#8 The Corrosion Of Metals In The Human Environment
1.Metal implant material
- •Implanted materials refer to implanted devices used to manufacture artificial organs inside the human body, small monitoring instruments, and treatment devices.
- •Materials used in plastic surgery to restore the human body, as well as materials for dentures and artificial tooth roots. Implant materials are also called biomedical materials.
- • Specific metal materials, organic polymer materials and ceramic materials. Ceramic materials, that is, bioceramic materials, are still immature. Among polymer materials, only ultra-high molecular weight polyethylene is currently the artificial joint plastic material commonly used in the world.
- • Metal implant materials are used in various places in the human body, and the human body has a highly corrosive environment, so the requirements for the corrosion resistance of metal implant materials are very important.
2.The characteristics of human environment
The human environment is made up of bodily fluids. Body fluid (physiological fluid) is an aerated solution of NaCl with a mass fraction of about 1%, a small amount of other salts, and organic compounds. Warm sea water is similar.
The environment of the human body is complex and living, and there is still a lack of sufficient understanding of its changing laws.
- Various corrosion behaviors of metal implant materials may intertwine and affect each other in the human body environment.
- The human body is sensitive, and the implanted material is required to achieve good repair and treatment purposes, and should not have harmful effects on the surrounding tissues, blood and human body.
- Body fluid is a typical electrolyte solution, and the basic laws of electrochemical corrosion are fully applicable to the corrosion of implanted materials in the human body environment. Body fluids generally have a neutral pH value. Sometimes geometric reasons or physiological conditions limit the supply of oxygen, and local body fluids become weakly acidic, containing H+, and implant materials are prone to pitting.
- Corrosion of metal implant materials belongs to oxygen depolarization corrosion, and its cathodic process:
Anodic process:
- The anode reaction of the microbattery is a dissolution reaction in which the metal loses electrons, resulting in the migration of metal ions. Metal ions such as cobalt, chromium, nickel, molybdenum, and titanium exist in the body fluid of patients with implanted metal parts and the tissues around the implanted parts. Whether they can cause metabolic abnormalities, interfere and be toxic to the human body is closely related to the corrosion resistance and biocompatibility of implant materials.
- The possible corrosion forms of implanted materials are basically the same as those of industrial metal materials. Corrosion is more harmful because it occurs in a specific human environment.
3.Corrosion forms that may occur in the human environment
1) Uniform corrosion
- In the human body, the loss of structural strength due to corrosion and thinning of implant materials is not the main problem. The problem is the biocompatibility of uniform corrosion products, which increases the suffering of patients and even endangers their lives.
- The uniform corrosion rate of metal implant materials in the human body is 2~3 orders of magnitude lower than that of general industrial materials. Uniform corrosion occurs on a large area, and the amount of metal ions entering human tissue is still considerable. The requirements for uniform corrosion resistance of metal implant materials are higher, and the annual thickness loss rate should not be greater than 0.254 μm. Because the passivation performance of titanium is very good. So it is satisfactory in surgical implant material.
2) Pitting corrosion
- See Section 3.2 for pitting corrosion conditions and damage. The implanted metal materials include stainless steel, cobalt-based, and iron-based alloys, all of which are easily passivated alloys and have passivation films. The corrosion resistance of stainless steel used in the human body is not satisfactory. The forced extraction rate of cobalt-chromium alloy implants was 3%, and there was no obvious pitting corrosion, while the pitting corrosion of 316 stainless steel was obvious.
- Studies on the pitting tendency of simulated physiological saline solution show that the pitting tendency of titanium alloy and cobalt-chromium-molybdenum alloy is very small, while the pitting tendency of stainless steel is large. Stainless steel containing Mo is resistant to pitting corrosion, but pitting corrosion will also occur if the Mo content is insufficient. For large bearing capacity and skeletal components, it is best to use pitting-resistant stainless steel and titanium alloys.
3) Galvanic corrosion
- Galvanic corrosion is especially important in implants consisting of multiple parts.
- If different materials are selected (potential difference), galvanic corrosion will easily occur.
- Such as bone plates and screws.
- Galvanic corrosion may also occur between surgical instruments and implant materials. Galvanic corrosion can also occur when metal shavings come into contact with the same material that has not been strongly deformed.
4) Crevice corrosion
Multiple-part implants, especially bone plates and screws, are subject to crevice corrosion, and crevice corrosion of stainless steel implants is an important corrosion phenomenon. Of the multi-part implants retrieved, 50% suffered from crevice corrosion. The forced take-out rate shows that crevice corrosion is second only to uniform corrosion.
5) Wear corrosion, intergranular corrosion
- Abrasion corrosion is the combined result of surface wear and corrosion environment caused by repeated, relative sliding between implanted devices.
- Intergranular corrosion is the most common corrosion form of stainless steel, and its damage is quite serious.
- The sensitization temperature of the material must be avoided in the process of making medical stainless steel implants.
- The mass fraction of carbon is reduced to less than 0.03%, which can eliminate the intergranular corrosion of stainless steel. According to the ISO832/1 international standard, the current medical stainless steel has a mass fraction of C lower than 0.03%.
6) Corrosion fatigue
- The fracture phenomenon of metal materials under the joint action of alternating stress and medium is corrosion fatigue.
- For implants used in human lower limbs, especially hip implants, corrosion fatigue resistance is crucial.
- Corrosion fatigue cracks occur from the surface of the implanted device, and the shot peening treatment of the implanted device can improve the fatigue life.
- Cast alloys are sensitive to corrosion fatigue, and wrought alloys have been clinically shown to be much less susceptible to corrosion fatigue fracture.
- The method of hot-pressing and forging is given priority to the high-stress implanted devices.
4.Commonly used metal implant materials
Implant Material Requirements
- The complexity of the human environment is almost impossible to simulate. The corrosion resistance requirements for implant materials are higher.
- There are three main requirements for implant materials: biocompatibility of materials with the human body, corrosion resistance in the environment of the human body, and mechanical properties of implant materials.
- Two aspects of biocompatibility: one is the effect of human tissue on implant materials, that is, the corrosion, fracture, and failure of implant materials; the other is the effect of implant material corrosion products and wear products on human tissues.
- Commonly used metal implant materials include stainless steel, cobalt-chromium-molybdenum alloy and titanium alloy.
